Importance of Transition Metals in Mammalian Development
The Lab
Transition metals and cell differentiation
My laboratory investigates the biological roles of transition metals, including copper (Cu), iron (Fe), zinc (Zn), and manganese (Mn), in mammalian cell biology, with particular emphasis on development, metabolism, and disease. These metals are essential micronutrients that function as cofactors for enzymes required for energy production, redox homeostasis, signal transduction, mitochondrial function, and tissue maturation. Because free metals can catalyze toxic reactions, cellular metal homeostasis is tightly regulated through high-affinity chelation, specialized transport systems, and metal-sensing transcriptional regulators that ensure precise spatial and temporal distribution.
A central focus of our work is understanding how metals are acquired, trafficked, and allocated to specific subcellular compartments and target proteins, and how these processes influence normal growth and differentiation. Although eukaryotic genomes encode a diverse array of metal transporters and metalloproteins whose biochemical properties are relatively well characterized, far less is known about the regulatory networks that control their expression, metal specificity, functional redundancy, and integration with broader signaling pathways. Using both skeletal muscle systems (including normal and Menkes disease murine models) and cultured cancer cellular models, we examine how dysregulated metal homeostasis contributes to altered cell fate decisions and disease progression.
Research Focus
Copper: one ion, different cellular destinations
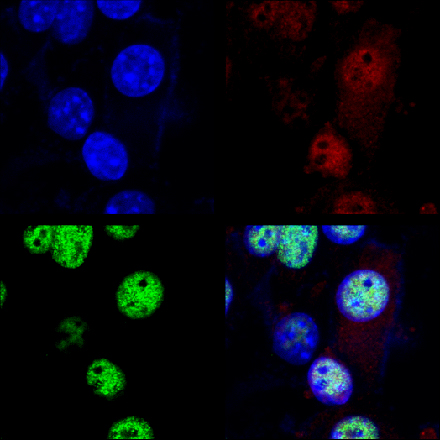
My lab conducts systematic studies that combine a variety of molecular and cell biology techniques into biological models, including established cell lines and primary cultures. We incorporate diverse biochemistry techniques and combine with high resolution cutting edge synchrotron-based X-ray fluorescence spectroscopy. In particular, we study skeletal muscle differentiation as muscle present an elevated intrinsic need for transition metals like Cu for proper function. This ion is required for mitochondrial energy production as a fundamental component of cytochrome c oxidase which is elevated during the course of differentiation. We hypothesize that the proper cellular distribution of Cu+ has a leading role in the differentiation of the muscle lineage. We have evidence that support different cellular roles for Cu in addition to energy production. Moreover, we hypothesize that diverse devastating myopathies are associated with Cu deficiencies at different levels, from mitochondrial Cu-transport and function to general cellular failure in Cu homeostasis and gene regulation. We hope to provide novel molecular mechanisms that help to understand the basis of muscular phenotypes observed in mitochondrial myopathies and also in Menkes’ and Wilson’s disease patients.
Publications

Latest publications from the lab:
CuiT is a Cu importer required for metal homeostasis in Salmonella enterica. 2026. Zhenzhen Zhao, Karla F. Díaz Rodríguez, Andrea A. E. Méndez, Lisandro M. Sommer, Pedro Mendes, Fernando C. Soncini, Susana K. Checa, Teresita Padilla-Benavides* & José M. Argüello*. BioRxiv. 10.64898/2026.01.22.701185
Reciprocal regulation between the protein arginine deiminases and mSWI/SNF chromatin remodelers controls skeletal muscle differentiation and regeneration. 2026. Monserrat Olea-Flores, Tapan Sharma, Anand Parikh, Leonard Barasa, Sarah Hachmer, F. Jeffrey Dilworth, Teresita Padilla-Benavides, Paul Thompson & Anthony N Imbalzano. BioRxiv. 10.64898/2026.01.20.700682v1
Tceal7 is a BRG1-regulated target of calcineurin signaling that promotes myoblast differentiation. Sandhya Yadav, Tapan Sharma, Teresita Padilla-Benavides* & Anthony N. Imbalzano*. 2026. BioRxiv. 10.64898/2026.01.20.70046
Cell Death Induced by Homoisoflavonoid Brazilin and its Semi-synthetic Derivates on MDA-MB-231 and MCF7 Breast Cancer Cell Lines .2026. Miriam Zuñiga-Eulogio, Michael Quinteros, Alberto Hernández-Moreno, Tadeo Hernández-Moreno, Tapan Sharma, Mario Ordóñez, Carla Coste-Sanchez, Teresita Padilla-Benavides* & †Napoleón Navarro-Tito. WE DEDICATE THIS MANUSCRIPT TO OUR COLLEAGUE DR. NAPOLEON NAVARRO-TITO. BioRxiv. 10.64898/2026.01.09.698710
Cysteine rich intestinal protein 2 links copper homeostasis to translational regulation in primary myoblasts 2025. Odette Verdejo-Torres & Teresita Padilla-Benavides*. microPublication Biology. 10.17912/micropub.biology.001889.
How undergrad research catalyzes scientific career. 2025. Antonio Rivera, Julissa Cruz-Bautista & Teresita Padilla-Benavides*. ASBMB Today (member magazine of the American Society for Biochemistry and Molecular Biology).
Editorial: Repurposed Drugs Targeting Cancer Signaling Pathways: Clinical Insights to Improve Oncologic Therapies Volume II. Alma D., Campos-Parra, Gerardo Leyva-Gómez, & Teresita Padilla-Benavides. 2025. Frontiers in Oncology – Molecular and Cellular Oncology. 15 https://doi.org/10.3389/fonc.2025.1628842
The PBAF chromatin remodeling complex contributes to metal homeostasis through Mtf1 regulation. Nicholas Carulli, Emma E. Johnston, David C. Klein, Odette Verdejo-Torres, O., Anand Parikh, Antonio Rivera, Michael Quinteros, Aidan T. Pezacki, Chrostopher J. Chang, Sarah J. Hainer, and Teresita Padilla-Benavides*. 2025. Preprint at BioRXiV: https://doi.org/10.1101/2025.04.12.648552.
Revolutionizing ovarian cancer therapy by drug repositioning for accelerated and cost-effective treatments. 2025. Edgar Y. Villegas-Vazquez, Francisco P. Marín-Carrasco, Octavio D. Reyes-Hernández, Andrea S. Baez-Gonzalez, Lilia P. Bustamante-Montes, Teresita Padilla-Benavides, Laura I. Quintas-Granados, and Gabriela Figueroa-González. Frontiers in Oncology – Molecular and Cellular Oncology. 14 https://doi.org/10.3389/fonc.2024.1514120
Cysteine Rich Intestinal Protein 2 is a copper-responsive regulator of skeletal muscle differentiation and metal homeostasis. 2024. Odette Verdejo-Torres, David C. Klein, Lorena Novoa-Aponte, Jaime Carrazco-Carrillo, Denzel Bonilla-Pinto, Antonio Rivera, Arpie Bakhshian, Fa’alataitaua Fitisemanu, Martha L. Jiménez-González, Lyra Flinn, Aidan T. Pezacki, Antonio Lanzirotti, Luis Antonio Ortiz-Frade, Christopher J. Chang, Juan G. Navea, Crysten Blaby-Haas, Sarah J. Hainer & Teresita Padilla-Benavides*. Preprint at Biorxiv: https://www.biorxiv.org/content/10.1101/2024.04.10.588959v1. PLOS Genetics 20(12): e1011495. https://doi.org/10.1371/journal.pgen.1011495
Emerging perspectives of copper-mediated transcriptional regulation in mammalian cell development. 2024. Fa’alataitaua Fitisemanu, & Teresita Padilla-Benavides. Metallomics, Volume 16, Issue 10, https://doi.org/10.1093/mtomcs/mfae046
Muscle-Specific Pyruvate Kinase Isoforms, Pkm1 and Pkm2, Regulate Mammalian SWI/SNF Proteins and Histone 3 Phosphorylation During Myoblast Differentiation. 2024. Monserrat Olea-Flores, Tapan Sharma, Odette Verdejo-Torres, Imaru DiBartolomeo, Paul R. Thompson, Teresita Padilla-Benavides & Anthony N. Imbalzano. Biorxiv; https://www.biorxiv.org/content/10.1101/2024.04.10.588959v1
Single nucleotide polymorphisms and Zn transport by nuclear ZIP11 shape cancer phenotypes in HeLa cells. 2024. Elizabeth Y. Kim, Odette Verdejo-Torres, Karla Diaz-Rodriguez, Farah Hasanain, Leslie Caromile & Teresita Padilla-Benavides*. Biorxiv; https://doi.org/10.1101/2023.08.12.553076. Metallomics, mfae006. https://doi.org/10.1093/mtomcs/mfae006
Differential requirements for different subfamilies of the mammalian SWI/SNF chromatin remodeling enzymes in myoblast differentiation. 2023. Teresita Padilla-Benavides*, Monserrat Olea-Flores, Tapan Sharma, Sabriya A. Syed, Hanna Witwicka, Miriam D. Zuñiga-Eulogio, Kexin Zhang, Napoleon Navarro-Tito & Anthony N. Imbalzano*. International Journal of Molecular Sciences. 24 (14), 11256; https://doi.org/10.3390/ijms241411256
Functional effect of indole-3 carbinol in the viability and invasive properties of cultured cancer cells. Andrea S. Baez-Gonzalez, Jaime A. Carrazco-Carrillo, G. Figueroa-Gonzalez, L.I Quintas-Granados, Teresita Padilla-Benavides* & Octavio Reyes-Hernandez*. 2023. Biochemistry and Biophysics Reports, Latin America special edition. https://doi.org/10.1016/j.bbrep.2023.101492
Heterogenous nuclear ribonucleoprotein (hnRNP) A2/B1 regulates the abundance of the copper-transporter ATP7A in an isoform dependent manner. 2022. Cat McCann, Nesrin M. Hasan, Teresita Padilla-Benavides, S. Roy & Svetlana Lutsenko. Frontiers in Molecular Biosciences – Cellular Biochemistry. Research topic: Novel Approaches to Study Metals in Molecular Biology
The Oncopig as an emerging model to investigate copper regulation in cancer. 2022. Alyssa Carlson, Jaime Carrazco-Carrillo, Aaron Loder, Lobna Elkhadragy, Kyle M. Schachtschneider & Teresita Padilla-Benavides*. International Journal of Molecular Sciences. 23 (22), 14012. https://doi.org/10.3390/ijms232214012
The mitochondrial Cu+ transporter PiC2 (SLC25A3) is a target of MTF1 and contributes to the development of skeletal muscle in vitro. 2022. Cat McCann, Michael Quinteros, Ifeoluwa Adelugba, Marcos N. Morgada, Aida R. Castelblanco, Emily J. Davis, Antonio Lanzirotti, Sarah J. Hainer, Alejandro J. Vila, Juan G. Navea & Teresita Padilla-Benavides*. Frontiers in Molecular Biosciences – Cellular Biochemistry. Research topic: Novel Approaches to Study Metals in Molecular Biology. https://doi.org/10.3389/fmolb.2022.1037941
Oxidative reactions catalyzed by hydrogen peroxide produced by Streptococcus pneumoniae and other streptococci cause the release and degradation of heme from hemoglobin. 2022. Babek Alibayov, Anna Scasny, Faidad Khan, Aidan Creel, Perriann Smith, Ana G. Jop Vidal, Fa’alataitaua M. Fitisemanu, Teresita Padilla-Benavides, Jeffrey Weiser, and Jorge E. Vidal. https://doi.org/10.1128/iai.00471-22
Surface tension of model tissues during malignant transformation and epithelial–mesenchymal transition. Irène Nagle, Alain Richert, Michael Quinteros, Sébastien Janel, Henry Debost, Véronique Thevenet, Claire Wilhelm, Céline Prunier, Frank Lafont, Teresita Padilla-Benavides, Mathieu Boissan & Myriam Reffay. 2022. Frontiers in Cell and Developmental Biology. Cell Adhesion and Migration section. Special topic on Mechanical and Structural Phenotypes of Cells and Extracellular Matrices Govern Cell Adhesion and Migration. https://doi.org/10.3389/fcell.2022.926322
ZIP11 Regulates Nuclear Zinc Homeostasis in HeLa Cells and Is Required for Proliferation and Establishment of the Carcinogenic Phenotype. 2022. Monserrat Olea-Flores, Julia Kan, Alyssa Carlson, Sabriya A Syed, Cat McCann, Varsha Mondal, Cecily Szady, Heather M Ricker, Amy McQueen, Juan G Navea, Leslie A Caromile & Teresita Padilla-Benavides*. Frontiers in Cell and Developmental Biology. Cellular Biochemistry section. Special topic on Bioinorganic Chemistry of Metals in Cell Function and Disease. https://doi.org/10.3389/fcell.2022.895433
Differential requirements for different subfamilies of the mammalian SWI/SNF chromatin remodeling enzymes in myoblast cell cycle progression and expression of the Pax7 regulator. Teresita Padilla-Benavides*, Monserrat Olea-Flores, Yaje Nshanji, May T. Maung, Sabriya A. Syed & Anthony N. Imbalzano. 2022. BBA – Gene Regulatory Mechanisms. https://doi.org/10.1016/j.bbagrm.2022.194801
Padilla-Benavides’ trainees
*Correspondence
Funding

1R01AR077578 Mechanisms of Cu-binding factors to promote myogenic gene expression

